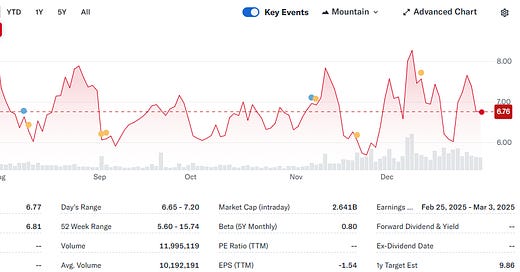
Recursion Pharmaceuticals is a TechBio company combining computational biology, machine learning, and high-throughput phenotyping via heavy automation to accelerate drug discovery and development. The company, led by CEO Chris Gibson, went public in 2021 and has positioned itself as a leader in leveraging AI-driven platforms to identify and optimize drug candidates.
Current programs from in-licensing
Recursion Pharmaceuticals' initial strategy included in-licensing four drug candidates to establish a clinical-stage pipeline while concurrently building their AI-driven drug discovery platform. These programs, brought in from external sources, were intended to demonstrate Recursion's ability to progress drugs through clinical development.
1. REC-994 (Cerebral Cavernous Malformation - CCM):
Indication: Aimed at treating cerebral cavernous malformation, a rare vascular disorder characterized by the development of abnormal blood vessels in the brain and spinal cord. The capillaries fill with blood and stretch, thereby creating cavernous spaces. Some patients experience headaches, seizures, or visual and hearing disturbances. Cerebral hemorrhage may also occur.
Origin: In-licensed to Recursion for development.
Status: Initial Phase 2 clinical trials were conducted; however, the program's progress has been disappointing due to weak efficacy data. This has raised concerns about the clinical viability of the candidate.
Challenges: CCM is a challenging therapeutic area with limited prior successes in drug development, and the disappointing results reflect these difficulties.
2. REC-2282 (Neurofibromatosis Type 2 - NF2):
Indication: Targeted neurofibromatosis type 2, a rare genetic disorder causing tumors to grow on nerves.
Origin: Another in-licensed candidate brought in to bolster Recursion's early clinical-stage pipeline.
Status: Phase 2 trials aimed to test efficacy and safety. There has been limited public disclosure about the trial outcomes, suggesting it remains under evaluation or has faced hurdles.
3. REC-4881 (Familial Adenomatous Polyposis - FAP):
Indication: Aimed to treat familial adenomatous polyposis, an inherited condition predisposing individuals to colorectal cancer due to the development of numerous polyps in the colon and rectum.
Origin: In-licensed from an external partner.
Status: Trial progress appears to have been limited, with no significant updates suggesting major advancements or successes.
4. REC-3599 (Traumatic Brain Injury - TBI):
Indication: Targeted traumatic brain injury, focusing on neurological repair and recovery post-injury.
Origin: Another candidate brought in through in-licensing agreements.
Status: Similar to REC-4881, limited data on clinical trial results or advancement has been shared, possibly reflecting setbacks or a deprioritization of this program.
Status of the 4 in-licensed programs:
Challenges:
One program was shelved early.
The CCM program yielded weak data.
Recursion's merger with Exscientia brought promising oncology programs, but concerns remain regarding their ability to execute early clinical development effectively.
Outlook: Their capability in this domain is under scrutiny, as past and current programs have not consistently met expectations.
Regulatory expertise
Another concern for RXRX is how well they will be able to navigate the regulatory process. So far they have had limited interaction with the FDA due to the early-stage nature of the initial programs. Their expertise here remains unproven, without any major commercial applications in the near future, which will become critical if and when they achieve success in their clinical trials.
Merger with Exscientia
In August 2024, Recursion Pharmaceuticals announced a definitive agreement to acquire Exscientia, a UK-based AI-driven drug discovery company, in an all-stock transaction valued at approximately $688 million. This strategic move aims to enhance Recursion's early-stage pipeline, particularly in oncology, by integrating Exscientia's precision chemistry and automated synthesis capabilities with Recursion's existing biological and chemical exploration platforms.
Recursion/Exscientia Oncology programs
The combined Recursion and Exscientia portfolio includes four promising oncology programs targeting key molecular pathways: CDK7, RBM39, MALT1, and LSD1. Each program focuses on areas with significant therapeutic potential and high market value, offering the possibility of blockbuster revenues if successful.
The CDK7 (Cyclin-Dependent Kinase 7) program centers on inhibiting a kinase involved in cell cycle regulation and transcriptional control. Dysregulation of CDK7 has been implicated in various cancers, including breast and lung cancer, where it drives unchecked cell proliferation. A drug targeting CDK7 has the potential to disrupt tumor growth and survival, representing a therapeutic avenue with strong clinical and commercial potential.
The RBM39 (RNA Binding Motif Protein 39) program focuses on a protein involved in RNA splicing and regulation. Dysregulation of splicing machinery is a hallmark of certain cancers, making RBM39 an attractive target. By modulating this protein, the program aims to selectively target cancer cells with aberrant splicing while sparing normal cells, offering a precision-medicine approach to treatment. This is a target gene with lower tractability, as there is less knowledge of the ligand and pocket, less evidence in the literature, but it also means that RXRX could establish a deeper moat in this area if they succeed in drugging the protein.
The MALT1 (Mucosa-Associated Lymphoid Tissue Lymphoma Translocation Protein 1) program is directed at an oncogenic driver involved in the activation of the NF-kB signaling pathway. MALT1 plays a critical role in B-cell malignancies, such as lymphomas and leukemias. Inhibiting MALT1 could suppress tumor growth and progression in these cancers. There are already approved drugs here, but again, it is still a risky target within the NF-kB pathway.
The KDM1A (also known as Lysine-Specific Demethylase 1) program targets an enzyme that regulates gene expression through epigenetic mechanisms. LSD1 is implicated in various cancers, including small-cell lung cancer and leukemia, where it promotes tumor cell proliferation and survival. By inhibiting LSD1, this program seeks to reprogram cancer cells and restore normal cell function. This is a more established target, lower risk for RXRX to come up with a drug against it.
REC-3964 C. difficile asset
REC-3964 is an orally administered small molecule developed by Recursion Pharmaceuticals for the treatment of recurrent C. difficile infection. Unlike traditional antibiotics, REC-3964 employs a targeted mechanism that inhibits the glucosyltransferase activity of toxin B produced by C. diff. in the gastrointestinal tract, thereby neutralizing the toxin's harmful effects without disrupting the beneficial gut microbiome.
In preclinical studies, REC-3964 demonstrated superior efficacy compared to bezlotoxumab, a monoclonal antibody therapy, in a hamster model of C. diff. infection. These promising results were further supported by a Phase 1 clinical trial involving healthy volunteers, where REC-3964 was well tolerated, with no serious adverse events reported.
Building on this foundation, Recursion initiated the Phase 2 ALDER clinical trial in October 2024. This multi-center, randomized study aims to assess the safety, tolerability, pharmacokinetics, and efficacy of REC-3964 in patients with recurrent C. diff. infection. The trial plans to enroll approximately 80 participants across the United States and Europe, administering oral doses of either 250 mg or 500 mg of REC-3964 over a 28-day treatment period.
Partnership with Tempus
In November 2023, Recursion Pharmaceuticals entered into a multi-year strategic collaboration with Tempus, a leader in artificial intelligence and precision medicine.
This partnership grants Recursion access to Tempus' extensive library of de-identified, multimodal data, enhancing Recursion's capabilities in biomarker-driven therapeutic development. By integrating Tempus' patient-centric oncology data with its own multimodal biological and chemical datasets, Recursion aims to accelerate the discovery and development of personalized oncology therapeutics. As part of the agreement, Recursion committed to annual payments totaling up to $160 million over five years. This collaboration underscores both companies' commitment to leveraging data-driven approaches to advance precision medicine.
Financial Management
Status: Financial concerns are significant, as the company has spent heavily on platform development without substantial clinical success to date.
Challenges:
The merger with Exscientia was partly motivated by low cash reserves.
The company may need to reduce spending to remain solvent.
Outlook: Financial sustainability is a pressing issue, and future viability depends on generating strong clinical data to attract investment or partnership opportunities.
Commercial Sales:
Status: Commercialization is a distant prospect.
Outlook: The company needs successful clinical trials before commercial sales become relevant.
Strategic Positioning
Recursion has faced a rocky trajectory post-IPO, with clinical data and financial pressures raising concerns about its long-term viability.