Using Next-Generation Sequencing for personalized cancer vaccines
The BioNTech pancreatic cancer clinical trial as an example
What are Cancer Vaccines and what do they have to do with Next-Generation Sequencing?
BioNTech, Moderna, and CureVac are the three leading companies working on cancer vaccines based on personalized neoantigens. Even though the average population will know of BioNTech and Moderna for their COVID-19 work, the original business model was heavily centered around cancer therapies, which is what we will cover here.
BioNTech is a German biotechnology company that was founded in 2008. The company is best known for developing the COVID-19 vaccine, BNT162b2, which is co-developed with Pfizer. BioNTech is also working on a number of other cancer vaccines, including autogene cevumeran, which is being evaluated in clinical trials for the treatment of pancreatic cancer, colorectal cancer, and melanoma.
Moderna is a U.S. biotechnology company that was founded in 2010. The company is best known for developing the COVID-19 vaccine, mRNA-1273, which is also co-developed with Pfizer. Moderna is also working on a number of other cancer vaccines, including mRNA-1647, which is being evaluated in clinical trials for the treatment of solid tumors.
CureVac is a German biotechnology company that was founded in 2000. The company is working on a number of cancer vaccines, including CV7001, which is being evaluated in clinical trials for the treatment of melanoma.
Personalized cancer vaccines based on neoantigens are a promising new approach to cancer treatment. These vaccines are designed to stimulate the immune system to attack cancer cells by presenting them with neoantigens, which are proteins that are produced by cancer cells but not by normal cells.
The early results from clinical trials of personalized cancer vaccines are promising. In some cases, these vaccines have been able to shrink tumors or even cure cancer. We will discuss the results of the pancreatic cancer clinical trial by BioNTech here as an example of this type of work.
—
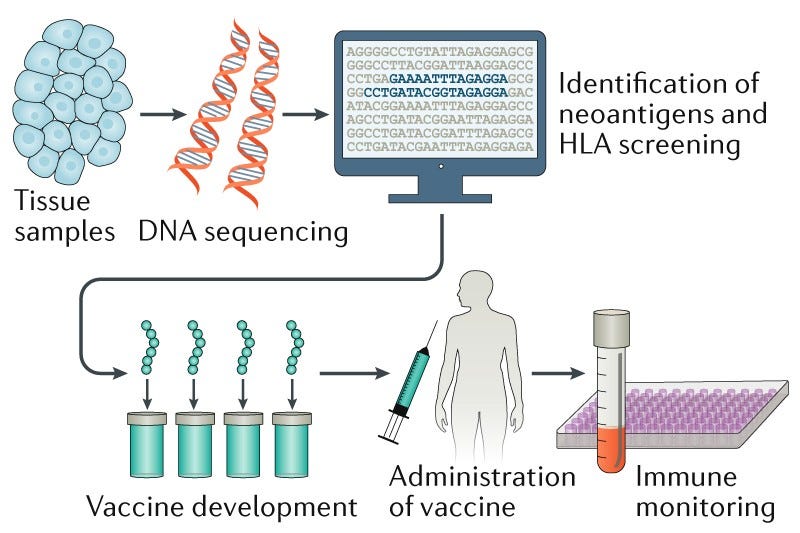
Next-Generation Sequencing (NGS) is a type of DNA sequencing that can be used to sequence the entire genome of a patient or tumor sample in a short period of time. This allows researchers to identify mutations that are present in the tumor cells but not in normal cells. These mutations can be used to design personalized cancer vaccines that can stimulate the immune system to attack the cancer cells.
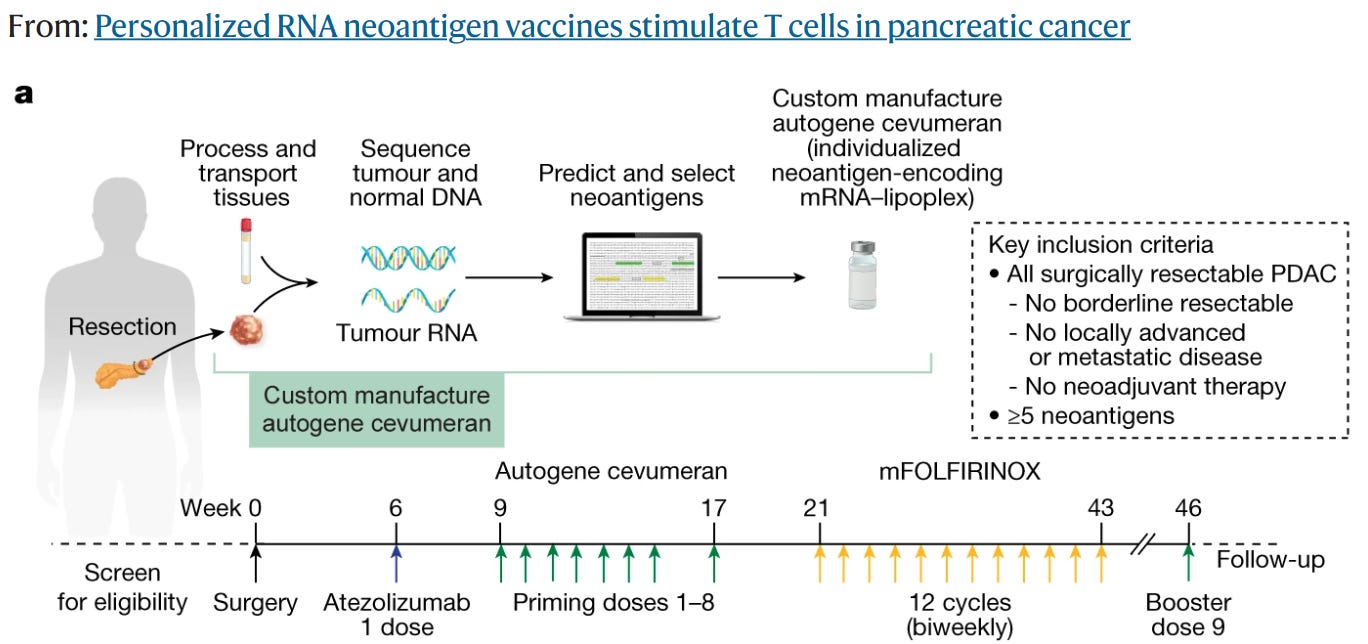
In the case of BioNTech, the company used the MSK-IMPACT panel to sequence the tumor DNA of patients with cancer. The MSK-IMPACT panel is a targeted tumor-sequencing test that covers 468 cancer genes. This panel was used to identify mutations in the tumor DNA that could be used to design personalized cancer vaccines.
The first step in the process is to enrich the tumor DNA for the targeted genes. This is done by using a hybridization-based protocol that binds the tumor DNA to a series of baits that cover the targeted genes. The enriched tumor DNA is then sequenced on an NGS instrument, in this case, they used an Illumina short-read instrument.
The sequence reads are aligned to the reference human genome and variants (SNVs, small indels, and copy number variants encompassing one or more exons) are called using bioinformatics tools. Variants can e classified according to, for example, the American College of Medical Genetics and Genomics guidelines. Only variants classified as pathogenic or likely pathogenic are reported and passed on to the next step.
The list of mutations identified in the MSK-IMPACT panel is then used to design the mRNA molecules used in the personalized vaccine. The mRNA molecules are designed to code for the neoantigens that are present in the tumor cells, but not present in healthy cells. When the vaccine is injected into the patient, the mRNA molecules are taken up by the cells and used to make the neoantigens. The presence of these “new proteins”, the neoantigens, then stimulate the immune system to attack them, thus attacking only the cancer cells.
It is important to note how crucial it is to design these mRNA molecules that will go into the cancer vaccine in a personalized manner, and the fact that they have to be different from any protein of protein fragment present in normal cells, otherwise the patient would either not respond to them or suffer an autoimmune response to the vaccine.
Why is it important to perform the Next-Generation Sequencing aspect with a fast turn-around time (TAT) and in a complete manner?
Designing personalized cancer vaccines targeting a patient's detected neoantigens requires a timely and comprehensive Next-Generation Sequencing (NGS) method. The use of NGS is crucial for accurately identifying all relevant mutations and ensuring effective targeting. Here's an explanation of why NGS is essential in this context:
1. Fast Turnaround Time (TAT): Personalized cancer vaccines are most effective when administered within weeks of starting treatment. This early intervention aims to target residual cancer cells or prevent the emergence of resistant clones. Therefore, a NGS method with a fast TAT is crucial to analyze the tumor DNA quickly and identify the neoantigens promptly. A shorter TAT enables timely vaccine development and administration.
2. Comprehensive Mutation Detection: Personalized cancer vaccines should encompass all relevant mutations in the patient's tumor DNA to maximize efficacy. If any mutations relating to cancer progression are missed during the NGS analysis, the vaccine might fail to target specific cancer cell populations, potentially leading to relapse or disease progression. A comprehensive NGS method is necessary to detect various types of mutations, including single nucleotide variants (SNVs), insertions/deletions (indels), and structural variants (SVs), e.g. from gene fusions.
3. Importance of Targeting all Mutations: By targeting all relevant mutations in the tumor, personalized cancer vaccines can induce an immune response against a broader spectrum of cancer cells. This approach reduces the likelihood of the cancer evolving from a clone of cells that contain mutations not targeted by the initial treatment. Comprehensive mutation profiling using NGS helps identify all potential neoantigens, increasing the chances of achieving a durable and effective immune response.
4. Individualized Vaccine Cocktail: The information obtained from NGS allows for the design of an individualized mRNA vaccine cocktail tailored to the patient's specific neoantigens. Each patient's tumor has a unique mutational profile, and NGS enables the identification of these personalized targets. This targeted approach enhances the vaccine's ability to stimulate an immune response against the specific mutations present in the tumor, improving treatment outcomes.
As an example of the above, the BioNTech pancreatic cancer clinical trial started injecting the mRNA vaccine 9 weeks after the patients were positively screened for eligibility in the trial (See below).
What was the main outcome of this clinical trial?
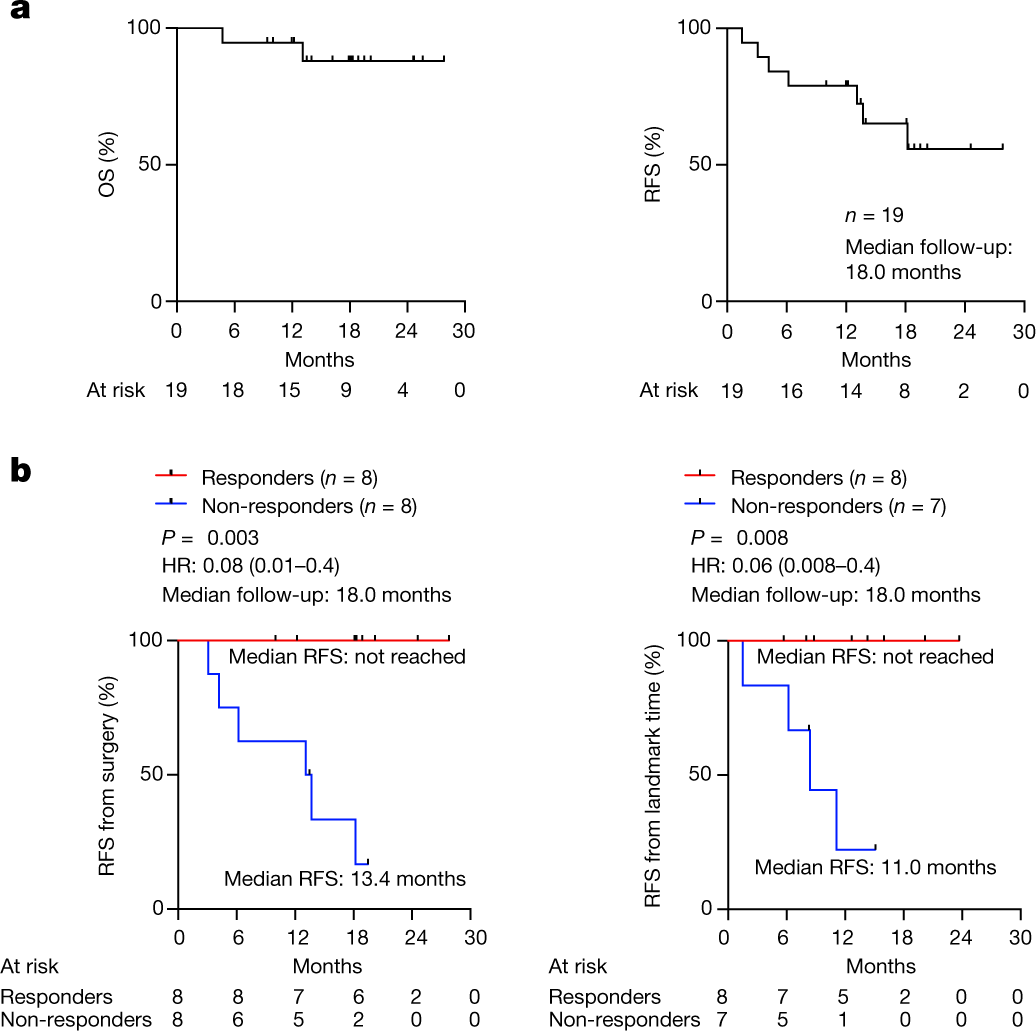
See the picture below for the “money shot”: patients that responded to the mRNA vaccine had a spectacular RFS, also known as "disease-free survival," which measures the length of time from the start of treatment or completion of treatment until the recurrence of the disease or the occurrence of new tumors. Similar to median OS, median RFS represents the midpoint in time when half of the participants in the trial or treatment group experienced disease recurrence or the occurrence of new tumors.
Which NGS companies are best positioned to ride the cancer vaccine wave?
Behind the curtain, I’ll hypothesize which NGS companies, such as ILMN 0.00%↑ Illumina, $ONT.L Oxford Nanopore, PACB 0.00%↑ PacBio, OMIC 0.00%↑ Singular Genomics, MGI Tech / Complete Genomics or Element Bio are best positioned to ride the wave of cancer vaccine design and therapies in the near future.